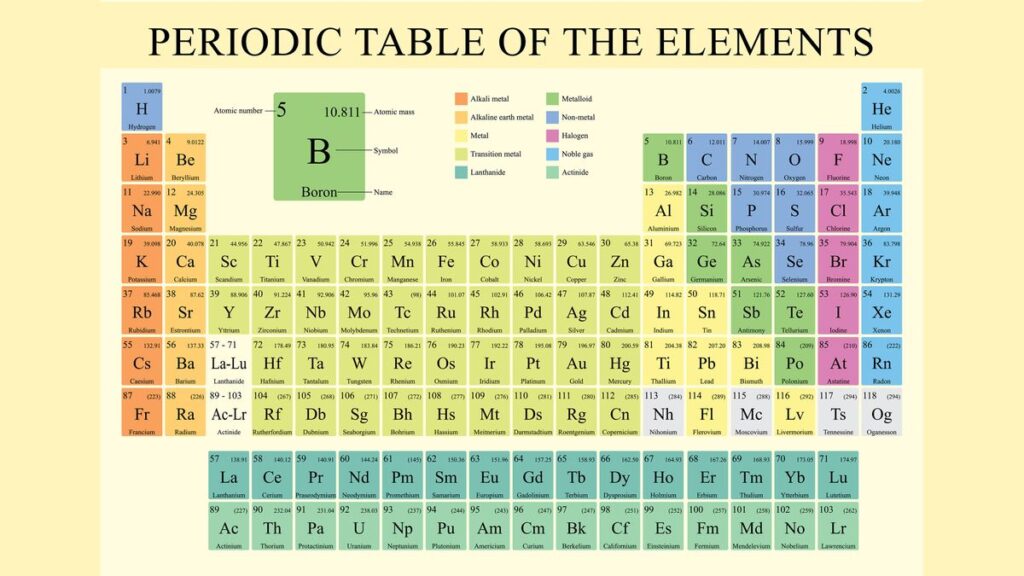
The Periodic Table of the Elements is more than just a list of the building blocks of matter; it’s a powerful tool that reveals patterns and relationships between elements, helping scientists predict chemical behavior. Its arrangement is the result of centuries of scientific discovery and understanding. This article dives deep into how the Periodic Table is organized, why it’s arranged the way it is, and how it continues to be a fundamental framework in chemistry.
1. The Historical Development of the Periodic Table
The arrangement of the Periodic Table didn’t happen overnight. Early chemists, like Antoine Lavoisier in the 18th century, attempted to classify elements based on their properties. However, the modern Periodic Table owes much to Dmitri Mendeleev, a Russian chemist who, in 1869, organized the elements based on their atomic masses and properties. Mendeleev left gaps in his table for elements that hadn’t been discovered yet, and remarkably, many of his predictions about these elements turned out to be accurate.
However, Mendeleev’s table wasn’t perfect. It was later refined when Henry Moseley in 1913 discovered that elements are better arranged by their atomic number (the number of protons in the nucleus) rather than their atomic mass. This revelation solidified the modern layout of the Periodic Table we use today.
2. Key Features of the Periodic Table’s Arrangement
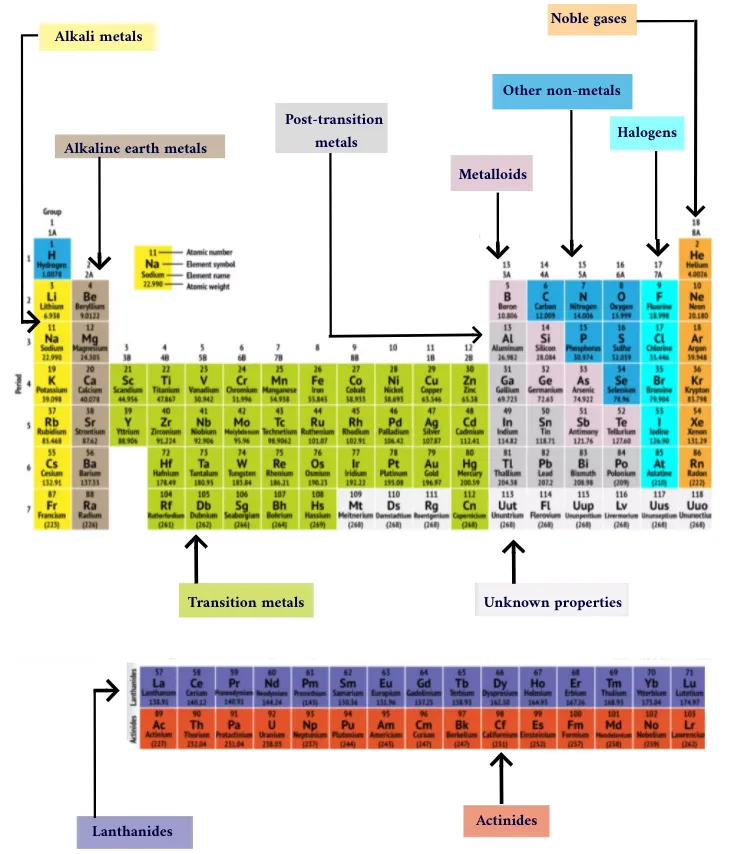
The Periodic Table is organized into rows, columns, and blocks, each reflecting specific properties of the elements. Let’s break it down:
a. Periods (Rows)
- The horizontal rows of the Periodic Table are called periods.
- Each period corresponds to the number of electron shells in an atom. For example, elements in the first period have one electron shell, while elements in the second period have two.
- As you move from left to right across a period, the atomic number increases by one for each element. This means each element has one more proton and one more electron than the element preceding it.
- A pattern in chemical properties emerges across a period. For instance, elements on the left side of a period (like metals) tend to lose electrons, while those on the right (non-metals) tend to gain electrons.
b. Groups (Columns)
- The vertical columns are known as groups or families.
- Elements in the same group have similar chemical properties because they have the same number of valence electrons (electrons in the outermost shell). These electrons determine how an element will react chemically.
- There are 18 groups in the modern Periodic Table. Each group is labeled either by numbers (1 to 18) or, in some older versions, with a combination of numbers and letters (e.g., 1A, 2A, etc.).
Some key groups include:
- Group 1: Alkali Metals (e.g., lithium, sodium, potassium) – These are highly reactive metals with one valence electron.
- Group 2: Alkaline Earth Metals (e.g., magnesium, calcium) – These metals have two valence electrons and are less reactive than alkali metals.
- Group 17: Halogens (e.g., fluorine, chlorine) – Highly reactive non-metals that gain electrons easily.
- Group 18: Noble Gases (e.g., helium, neon) – These gases are chemically inert because they have a full set of valence electrons, making them very stable.
c. Blocks
The Periodic Table is divided into four blocks based on the type of atomic orbital where the valence electrons are found. These blocks are:
- s-block: Groups 1 and 2 (and hydrogen and helium). Elements in this block have their outermost electrons in an s orbital.
- p-block: Groups 13 to 18. These elements have their outermost electrons in a p orbital.
- d-block: Transition metals in the middle of the table (Groups 3 to 12). Their valence electrons are found in d orbitals.
- f-block: These are the lanthanides and actinides, which are often displayed separately at the bottom of the table. Their electrons occupy f orbitals.
d. Metals, Non-Metals, and Metalloids
- The Periodic Table is also visually divided between metals, non-metals, and metalloids.
- Metals are found on the left side and center of the table. They are typically good conductors of heat and electricity, malleable, and shiny.
- Non-metals are on the right side of the table and have opposite properties: they are poor conductors, brittle in solid form, and lack luster.
- Metalloids, which sit along the staircase-like line between metals and non-metals, have properties of both and are used in semiconductors and other technologies.
3. Periodic Trends: What the Table Reveals About Elements
The organization of the Periodic Table helps reveal patterns, or periodic trends, which describe how certain properties change as you move across the table.
a. Atomic Radius
- The atomic radius refers to the size of an atom. As you move down a group, the atomic radius increases because each element has more electron shells.
- Conversely, as you move from left to right across a period, the atomic radius decreases. This happens because as more protons are added to the nucleus, they pull the electrons closer, making the atom smaller.
b. Electronegativity
- Electronegativity measures how strongly an atom attracts electrons in a bond.
- Electronegativity increases as you move from left to right across a period and decreases as you move down a group. The most electronegative element is fluorine, found in Group 17.
c. Ionization Energy
- Ionization energy is the energy required to remove an electron from an atom.
- Like electronegativity, ionization energy increases across a period and decreases down a group. It takes more energy to remove an electron from a smaller, more tightly held atom.
d. Metallic Character
- The metallic character of an element describes how readily it loses electrons, a property typical of metals.
- Metallic character increases as you move down a group and decreases as you move across a period from left to right.
4. Why the Periodic Table is Still Important Today
The Periodic Table is not just a historical curiosity. It remains essential in modern chemistry, physics, and even biology. By understanding how elements are arranged, scientists can:
- Predict the properties of new or unknown elements.
- Understand the behavior of materials in different environments.
- Explore chemical reactions more effectively, by knowing how elements interact based on their placement in the table.
With continued research in synthetic elements and materials science, the Periodic Table continues to grow and evolve.
FAQs: How the Periodic Table is Arranged
Q1. What is the Periodic Law?
- The Periodic Law states that the properties of elements are periodic functions of their atomic numbers. In other words, when elements are arranged by increasing atomic number, certain properties repeat at regular intervals.
Q2. Why are some elements placed at the bottom of the table (lanthanides and actinides)?
- The lanthanides and actinides are placed separately to keep the table more compact. They belong to periods 6 and 7 and would otherwise make the table too wide. Their properties are unique due to their f-electrons.
Q3. Why do elements in the same group behave similarly?
- Elements in the same group have the same number of valence electrons, which determine their chemical behavior. This similarity in electron configuration leads to similar reactivity and bonding characteristics.
Q4. How are synthetic elements arranged on the Periodic Table?
- Synthetic elements are placed in the same order as natural elements, based on their atomic number. These elements, usually created in laboratories, often fill gaps in the table and extend periods 6 and 7.
Q5. What is the most reactive element on the Periodic Table?
- The most reactive metal is francium (in Group 1), and the most reactive non-metal is fluorine (in Group 17). Francium reacts explosively with water, while fluorine aggressively seeks to gain electrons in reactions.
Q6. Will the Periodic Table ever change?
- Yes, the Periodic Table is dynamic. As new elements are synthesized and discoveries are made, the table will expand. Some theoretical models predict a future where the Periodic Table could include hundreds of elements.
Conclusion
The Periodic Table is a masterpiece of scientific organization, providing a comprehensive framework for understanding the behavior of elements. Its structure—from periods and groups to blocks and trends—allows scientists to predict how elements will interact in chemical reactions. As science advances, the Periodic Table will continue to evolve, but its foundational principles will remain a cornerstone of chemistry and beyond.